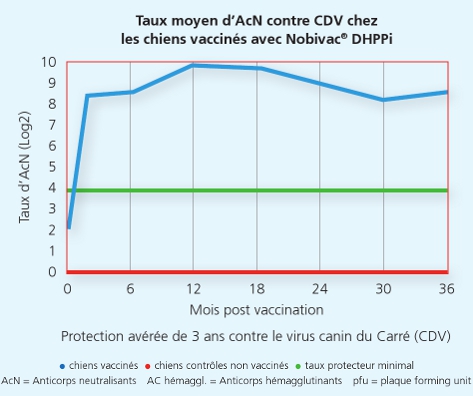
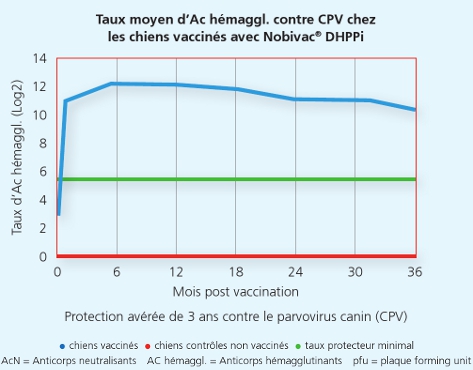
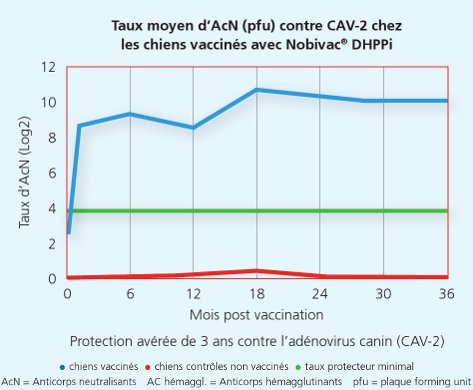
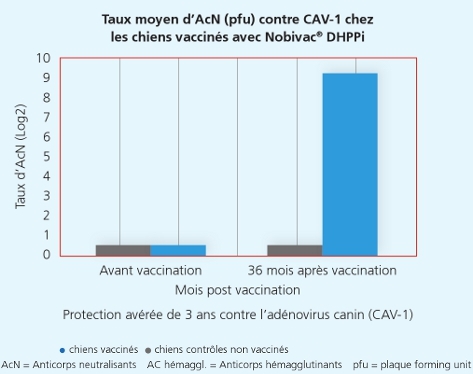
La souche Onderstepoort du Carré protège de la maladie et de la dissémination de virus infectieux du Carré
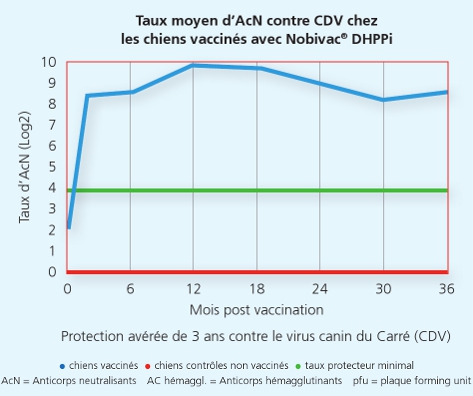
La souche Onderstepoort du Carré protège de la maladie et de la dissémination de virus infectieux du Carré 2
La souche Onderstepoort du Carré de MSD (auparavant Intervet) s’avère être un antigène vaccinal très fiable.
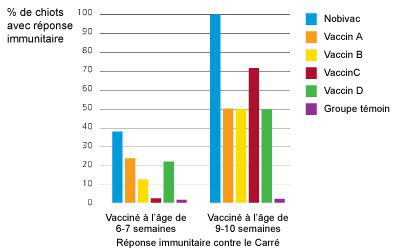
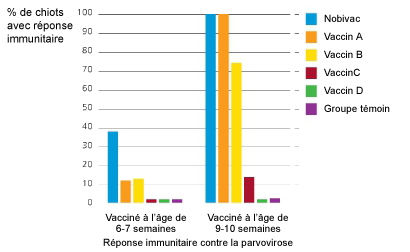
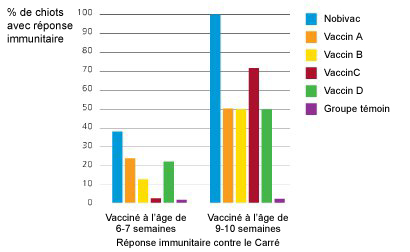
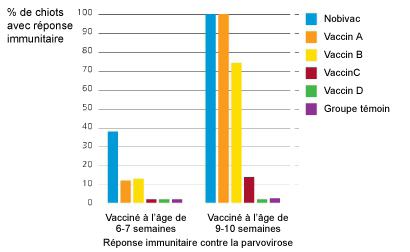
Dans une étude comparative réalisée aux Etats-Unis avec 5 vaccins disponibles sur le marché, Nobivac® DHPPi était le seul vaccin à immuniser 100% des chiots de 10 semaines vaccinés deux fois.
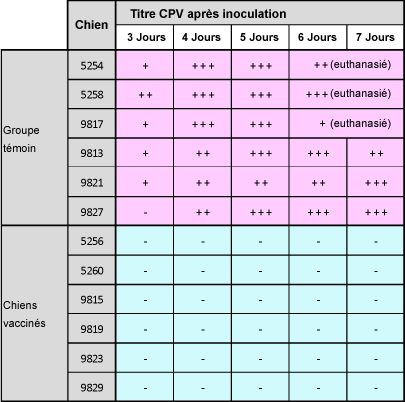
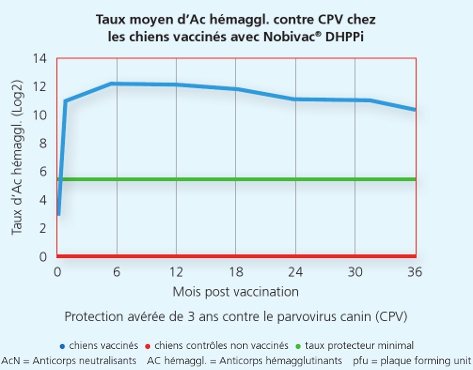
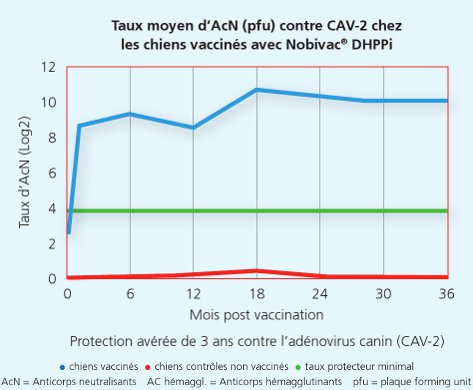
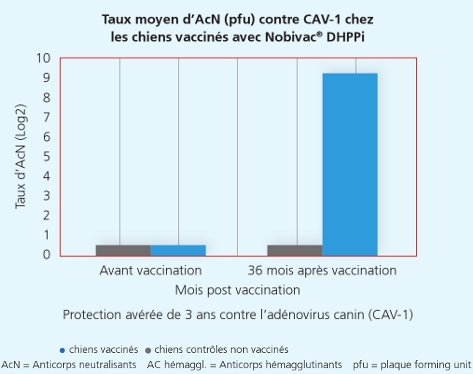
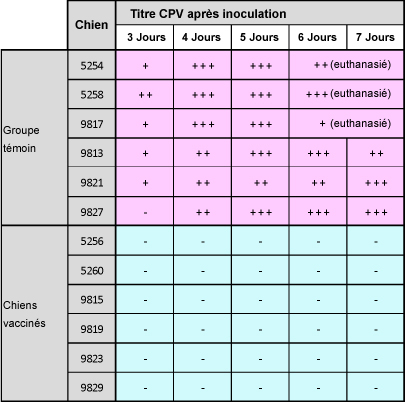
Une autre étude a démontré que la souche Onderstepoortprotège non seulement de la maladie, mais également contre la transmission de virus infectieux d’un animal à l’autre.1
- Data on file, reprint available on request at: MSD Animal Health GmbH, Weystrasse 20, 6006 Luzern
- Bergmann J.G.H.E.(1997): Comparative study with 5 different distemper vaccines. Veterinary Quarterly 19, 51
- Hoskins et al (1995): Challenge trial of a new attenuated canine parvovirus vaccine. J Vet Int Med, 9, 197
- Larson (2008): Do two canine parvovirus type 2 and 2b vaccines provide protection against the new type 2c variant? Vet Ther 5, 94-101
- Spibey et al (2008): Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet Microbiol 128, 48-55
- Institut für Viruskrankheiten und Immunprophylaxe (2010): Impfung von Katzen- und Marderartigen, Waschbären, Elefanten und Neuweltkameliden
- Barmettler R et al (2011): Assessment of exposure to Leptospira serovars in veterinary staff and dog owners in contact with infected dogs. JAVMA 238, 183-188
- Klaasen et al (2013). A novel tetravalent Leptospira vacterin protects against infection and shedding following challenge in dogs. Vet Rec 172, 181
- Ellis WA (2010): Control of canine leptospirosis in Europe: time for a change? Vet Rec 167, 602-605
- Gore et al (2005): Three year duration of immunology in dogs following vaccination against canine adenovirus type-1, canine parvovirus and canine distemper virus. Vet Ther 6, 5-14
- Abdelmagid et al (2004): Evaluation of the Duration of Immunity of a Canine Combination Vaccine against Virulent Parvovirus, Infectious Canine Hepatitis Virus, and Distemper Virus Experimental Challenges. Vet Ther 5, No 3, Fall 2004
- Brunner R. (1991): Immunkompetenz und Impfungen beim Hundewelpen. Collegium Veterinarium XXII, 102-109
- Hoskins J.D. et al (1995): Performance of a new generation canine parvovirus in Rottweiler puppies. Canine practice 22: 29-31
- Ohmori K et al (2007): Immunoblot analysis for IgE-reactive components of fetal calf serum in dogs that developed allergic reactions after non-rabies vaccination. Vet Imm 115: 166-171
- Gore T, Headley M, Raris R, et al (2005) Intranasal kennel cough vaccine protecting dogs from experimental Bordetella bronchiseptica challenge within 72 hours. Veterinary Record 156, 482-483
- Jacobs AA, Theleen RP, Jaspers R, et al (2005): Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Veterinary Record 157, 19-23
- Porter, C.J. et al (2008): Comparison of the ability of feline calicivirus (FCV) vaccines to neutralise a panel of current UK FCV isolates. Journal of Feline Medicine and Surgery (2008) 10, 32-40
- Gore et al (2006): Three-year duration of immunity in cats following vaccination against feline rhinotracheitis virus, feline calicivirus and feline panleukopenia virus. Vet Ther, 7 (3), 213-222
- Brunner C. et al (2006): Antibody induction after combined application of an adjuvanted recombinant FeLV vaccine and a multivalent modified live virus vaccine with chlamydial component. Vaccine, 24, 1838-1846
- Wills JM et al (1988): Prevalence of Chlamydophilapsittaci in different cat populations in Britain. JSAP, 327-339
- Hu et al (2006): Prevention of rabies virus infection in dog by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes and Infection 8, 1090-7
- Van de Zande et al (2009): Comments to ‘Comparison of antibody responses after vaccination with two inactivated rabies vaccines’. Vet Microbiol. 2009 Mar 4
- FAVN = Fluorescent Antibody Virus Neutralisation (Serumneutralisationstest)
- WHO Technical Report Series, No. 840, 1994, Annex 6
- Data on file. Intervet subject memo report 03R/0384: analysis of rabies serology data from UK