Mise en place rapide de l’immunité
Mise en place rapide de l’immunité
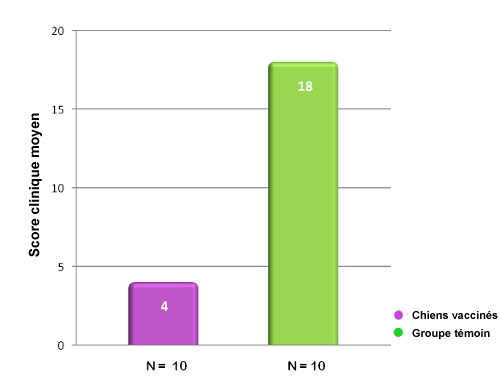
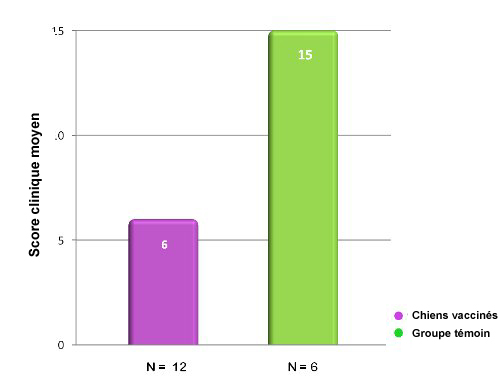
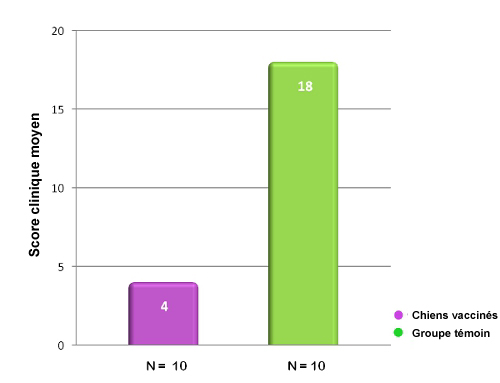
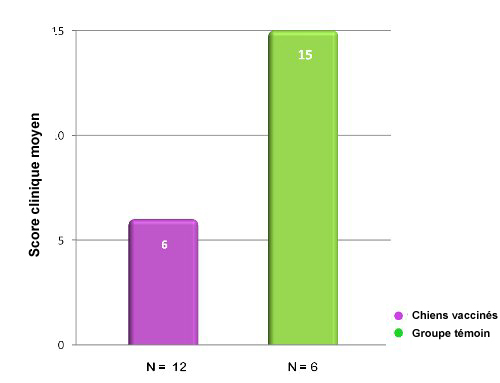
Dans cette étude portant sur une inoculation d’épreuve avec Bordetella bronchiseptica, les chiens vaccinés avec Nobivac® KC avaient,
72 h après la vaccination, un score clinique significativement plus bas que les animaux témoins non vaccinés.15
- Data on file, reprint available on request at: MSD Animal Health GmbH, Weystrasse 20, 6006 Luzern
- Bergmann J.G.H.E.(1997): Comparative study with 5 different distemper vaccines. Veterinary Quarterly 19, 51
- Hoskins et al (1995): Challenge trial of a new attenuated canine parvovirus vaccine. J Vet Int Med, 9, 197
- Larson (2008): Do two canine parvovirus type 2 and 2b vaccines provide protection against the new type 2c variant? Vet Ther 5, 94-101
- Spibey et al (2008): Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet Microbiol 128, 48-55
- Institut für Viruskrankheiten und Immunprophylaxe (2010): Impfung von Katzen- und Marderartigen, Waschbären, Elefanten und Neuweltkameliden
- Barmettler R et al (2011): Assessment of exposure to Leptospira serovars in veterinary staff and dog owners in contact with infected dogs. JAVMA 238, 183-188
- Klaasen et al (2013). A novel tetravalent Leptospira vacterin protects against infection and shedding following challenge in dogs. Vet Rec 172, 181
- Ellis WA (2010): Control of canine leptospirosis in Europe: time for a change? Vet Rec 167, 602-605
- Gore et al (2005): Three year duration of immunology in dogs following vaccination against canine adenovirus type-1, canine parvovirus and canine distemper virus. Vet Ther 6, 5-14
- Abdelmagid et al (2004): Evaluation of the Duration of Immunity of a Canine Combination Vaccine against Virulent Parvovirus, Infectious Canine Hepatitis Virus, and Distemper Virus Experimental Challenges. Vet Ther 5, No 3, Fall 2004
- Brunner R. (1991): Immunkompetenz und Impfungen beim Hundewelpen. Collegium Veterinarium XXII, 102-109
- Hoskins J.D. et al (1995): Performance of a new generation canine parvovirus in Rottweiler puppies. Canine practice 22: 29-31
- Ohmori K et al (2007): Immunoblot analysis for IgE-reactive components of fetal calf serum in dogs that developed allergic reactions after non-rabies vaccination. Vet Imm 115: 166-171
- Gore T, Headley M, Raris R, et al (2005) Intranasal kennel cough vaccine protecting dogs from experimental Bordetella bronchiseptica challenge within 72 hours. Veterinary Record 156, 482-483
- Jacobs AA, Theleen RP, Jaspers R, et al (2005): Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Veterinary Record 157, 19-23
- Porter, C.J. et al (2008): Comparison of the ability of feline calicivirus (FCV) vaccines to neutralise a panel of current UK FCV isolates. Journal of Feline Medicine and Surgery (2008) 10, 32-40
- Gore et al (2006): Three-year duration of immunity in cats following vaccination against feline rhinotracheitis virus, feline calicivirus and feline panleukopenia virus. Vet Ther, 7 (3), 213-222
- Brunner C. et al (2006): Antibody induction after combined application of an adjuvanted recombinant FeLV vaccine and a multivalent modified live virus vaccine with chlamydial component. Vaccine, 24, 1838-1846
- Wills JM et al (1988): Prevalence of Chlamydophilapsittaci in different cat populations in Britain. JSAP, 327-339
- Hu et al (2006): Prevention of rabies virus infection in dog by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes and Infection 8, 1090-7
- Van de Zande et al (2009): Comments to ‘Comparison of antibody responses after vaccination with two inactivated rabies vaccines’. Vet Microbiol. 2009 Mar 4
- FAVN = Fluorescent Antibody Virus Neutralisation (Serumneutralisationstest)
- WHO Technical Report Series, No. 840, 1994, Annex 6
- Data on file. Intervet subject memo report 03R/0384: analysis of rabies serology data from UK